-
Home
-
Products and Solutions
-
Keyto Global
-
News Center
-
Service Support
-
About Us
-
Contact Us
-
语言



Microfluidic technology has become a core enabler in life sciences, medical diagnostics, drug discovery, and environmental monitoring. Acting as the“lab on a chip,” microfluidic devices are reshaping industries with their precision and scalability.
At Keyto, we have dedicated more than a decade to advancing microfluidic chip design, production, and quality assurance. This article takes you inside our R&D labs, manufacturing facilities, and quality control systems, showing how Keyto turns innovative concepts into reliable solutions for global healthcare and scientific applications.
Microfluidic chip R&D is a complex balance of fluid dynamics, material science, and microelectronics. At Keyto, the engineering teams combine simulation, prototyping, and client collaboration to accelerate innovation.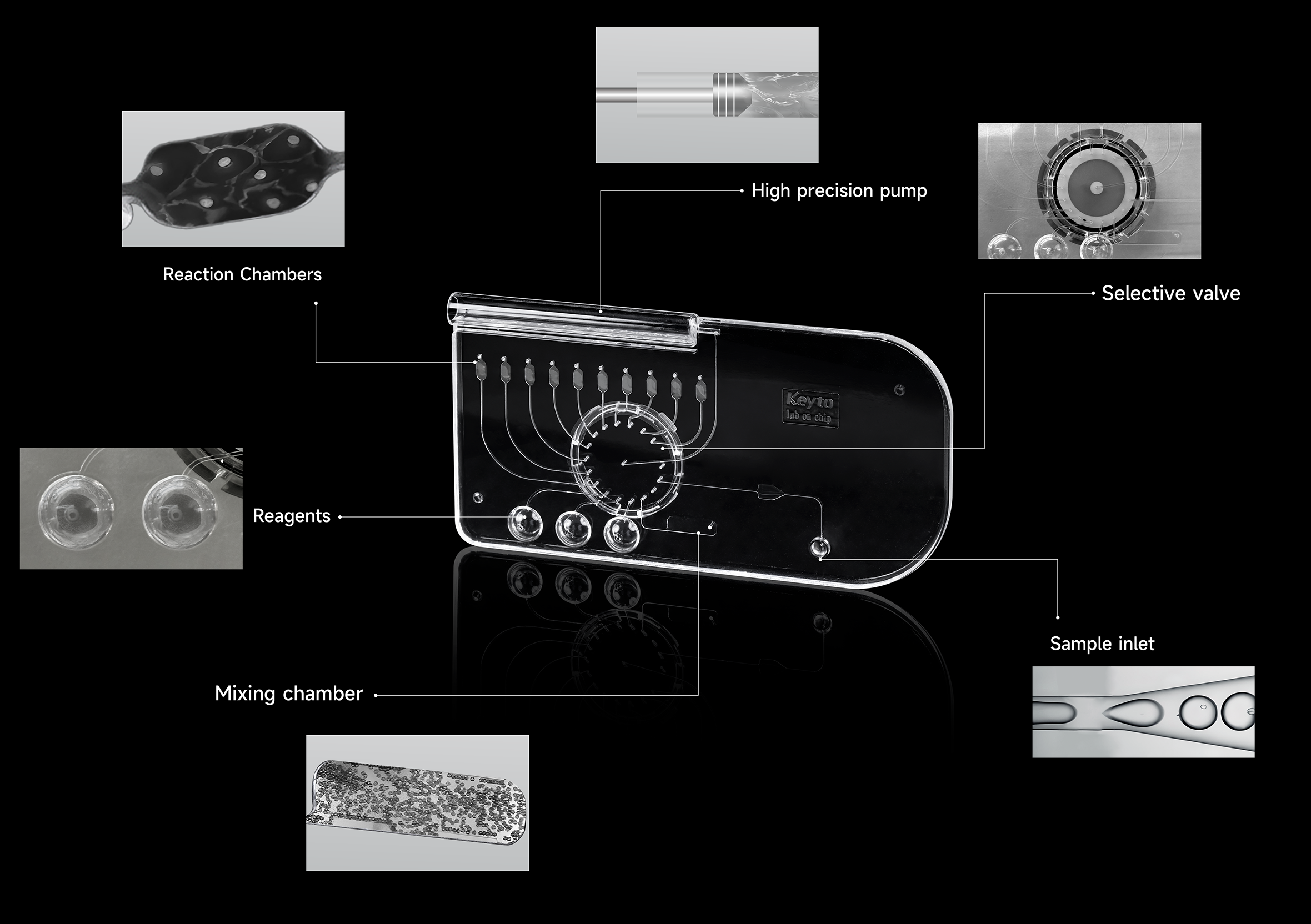
Keyto R&D follows a dual-driven approach: customer needs + deep research. For example, in clinical diagnostics where high sensitivity is critical, we use computational fluid dynamics (CFD) to simulate flow patterns in microchannels. Keyto also applys molecular dynamics modeling to identify surface-modification materials such as PMMA, PC, COC, and COP. These materials help balance low surface energy with high biocompatibility, ensuring sample purity and cell viability.
Keyto adopts an agile development model, enabling rapid prototype creation with micro-precision techniques. Prototypes are tested on client platforms using pressure sensors, fluorescence microscopy, or AI image analysis. Data is fed back to Keyto engineers, who quickly optimize the next iteration. This design–test–refine loop significantly shortens development cycles and improves application performance.
To overcome global supply chain restrictions, Keyto has developed self-reliant core technologies. We integrate valves and pumps directly into chips, minimizing mismatches between components and improving reliability. With over 100 patents and proprietary innovations, Keyto builds a strong technological moat for our partners.
Scaling microfluidic chips from research prototypes to industrial production requires precision and flexibility. Keyto’s automated production lines combine efficiency, repeatability, and customization.
Instead of relying on costly photolithography, Keyto applies a hybrid process of hot embossing + molecular bonding. This ensures microchannel accuracy while reducing costs to nearly one-third of traditional methods.
Keyto’s production system is modular and digital. Each process—molding, bonding, surface treatment—can be adjusted independently. With MES, ERP, and PLM integration, we achieve full traceability, fast response to customized orders, and secure protection of client intellectual property.
Keyto’s facilities combine CNC machining, ±5 μm injection molding, plasma bonding, and laser welding with process innovations such as thermal bonding, ultrasonic welding, and mold-flow analysis. Our surface engineering achieves Ra ≤ 0.02 μm polishing, micro-holes of Ø0.1–0.5 mm, optical transparency of ≥92%, and ultra-thin walls of 0.05 mm with aspect ratios up to 1:80.
We also ensure precise internal channel volume control of 50–200 μL (±1%), meeting the demands of chromatography, drug screening, PCR, and genetic sequencing applications.
Keyto approaches quality with the mindset of “zero defects”, ensuring that every chip delivered is consistent, reliable, and compliant with international standards. Our quality management system covers the entire product lifecycle, from raw material inspection to final testing under real-world conditions.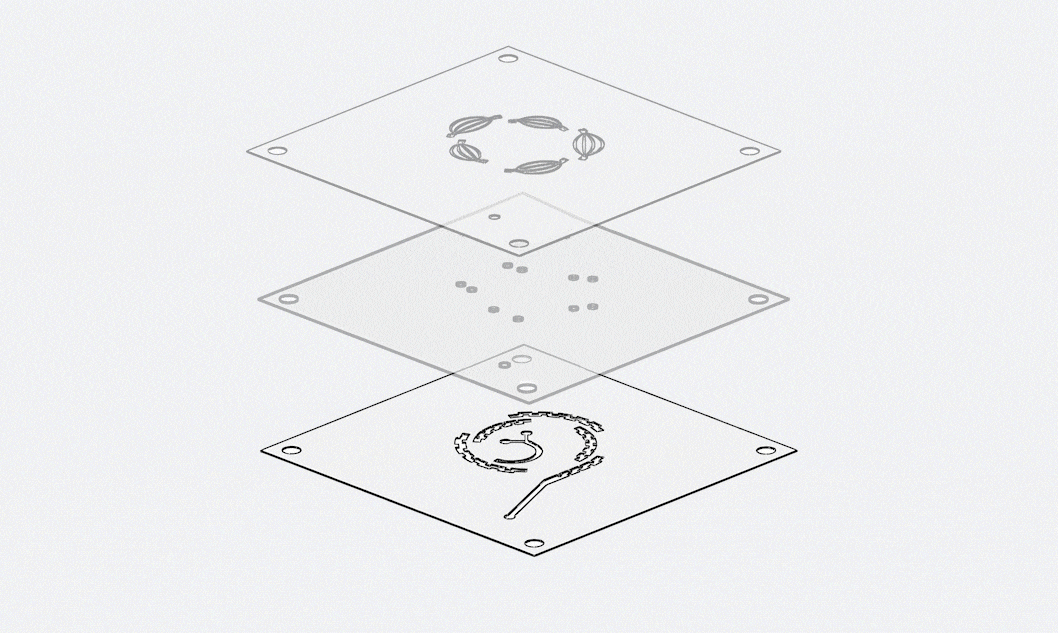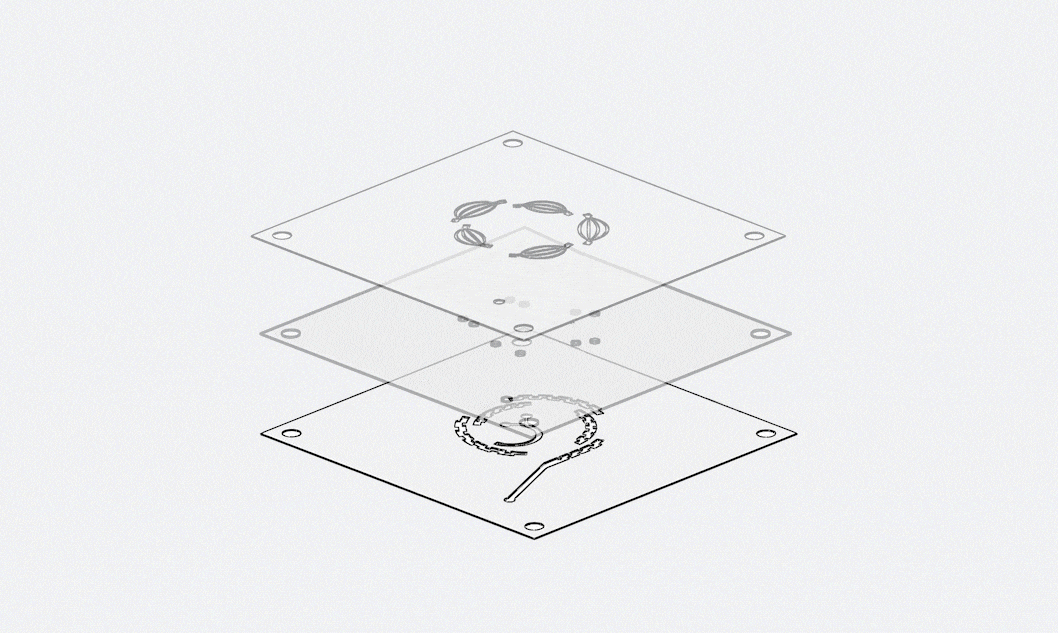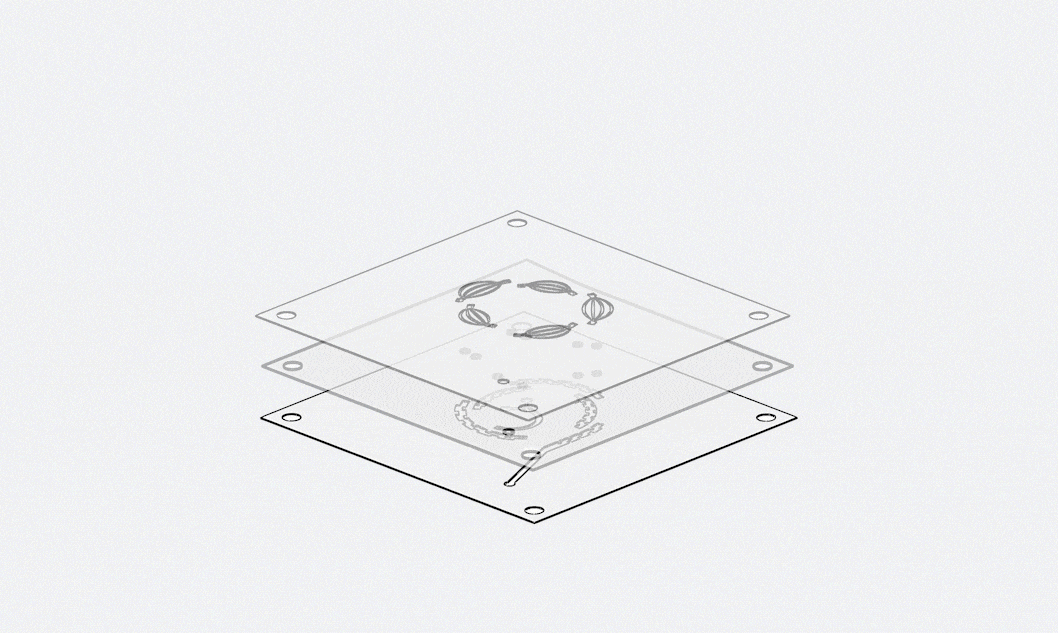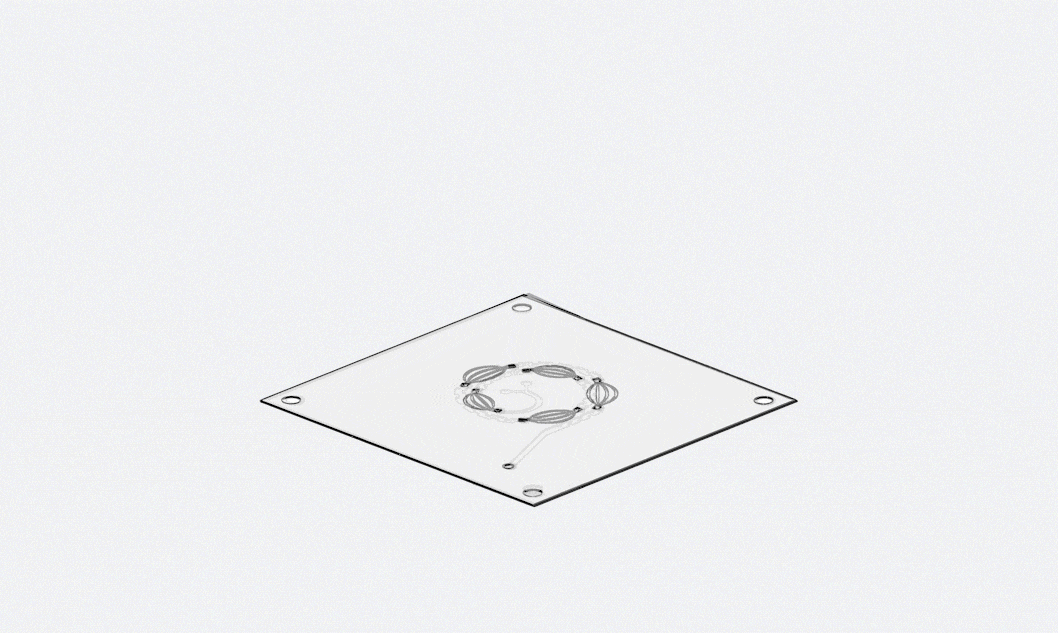
Quality begins with materials. All polymers and substrates undergo dual-batch inspections and full-parameter scanning before entering production. During manufacturing, we implement statistical process monitoring at critical steps such as injection molding. If any parameter deviates beyond the standard range, the system automatically halts production for correction. Once chips are completed, they are subjected to reliability testing that simulates client scenarios—including stress loads and environmental challenges—to guarantee that they will not leak, deform, or fail in real applications.
Quality improvement continues after delivery. Keyto maintains a customer-driven feedback system that includes tailored testing protocols, regular review meetings to refine processes, and dedicated on-site engineers for strategic clients. This ensures that our microfluidic chips consistently meet the demands of healthcare, life sciences, and environmental monitoring applications.
From concept design to industrial manufacturing and strict quality control, Keyto is committed to driving the future of microfluidics.
Keyto’s microfluidic chips are already used in health screenings for millions worldwide, from infectious disease detection to early cancer diagnostics. In environmental monitoring industry, portable microfluidic chips can detect heavy metals in water in realtime, contributing to sustainable development.
We believe that the “lab on a chip” will one day become part of everyday life—delivering precision, efficiency, and trust.