-
Home
-
Products and Solutions
-
Keyto Global
-
News Center
-
Service Support
-
About Us
-
Contact Us
-
语言


Healthcare, life sciences, and analytical testing are moving toward one shared goal: get reliable results faster, with less sample, less manual work, and fewer constraints on where testing can happen. That demand is driving the rapid adoption of microfluidic chips, also known as Lab-on-a-Chip platforms.
A microfluidic chip enables precise handling of tiny liquid volumes inside microchannels—making it possible to integrate multiple laboratory steps (sampling, mixing, reaction, separation, and detection) into a compact format. This is why microfluidics is widely viewed as a key enabler for portable diagnostics, scalable cartridge-based systems, and more automated testing workflows.
If you are exploring microfluidics for product development or assay migration, start here: microfluidic chips.
A microfluidic chip is a structured platform that routes liquids through micro-scale channels and chambers to execute controlled operations—often with higher consistency and less reagent use than traditional benchtop workflows. Microfluidics matters because it can improve both performance and deployability:
This is why microfluidic chips are increasingly used for portable diagnostics, integrated sample-to-answer systems, and scalable cartridge platforms.
A“pocket lab”is not a single component—it is an integrated system. Most microfluidic platforms rely on four functional layers:
To keep flow paths stable, many systems use integrated structures that reduce interfaces and leak risk. A common approach is to combine chip channels with robust packaging and manifold structures such as bonded manifolds, especially when you need scalable assembly and repeatable performance.
Precise routing requires reliable switching and isolation—particularly when assays include multiple reagents, wash steps, or timed reactions. Compact solenoid valves are frequently used to enable repeatable on/off control and consistent fluid handling.
Whether you need controlled aspiration, dispensing, or steady transport, pumping is central to stability. For compact systems and sampling workflows, pumps help maintain consistent flow rates and support automation-friendly designs.
The chip ultimately needs a readout interface—optical, fluorescence, electrochemical, or other modalities—depending on the assay. Microfluidics improves detection quality by stabilizing volumes, timing, and fluid contact surfaces.
Taken together, these elements turn a microfluidic chip into a reliable miniature lab platform rather than a fragile prototype.
Microfluidics is already proving its impact across healthcare, life sciences, and industrial testing. Below are practical application areas that show why microfluidic chips are adopted—not as a novelty, but as a performance and workflow upgrade.
Microfluidics is a natural fit for PCR workflows because it can reduce reaction volume and improve thermal responsiveness. In many designs, microchannels and microchambers make it easier to control reaction conditions—supporting faster cycling and more portable implementations.
For digital PCR (dPCR), microfluidic partitioning approaches can split a sample into many microreactors (e.g., microwells or droplets), enabling absolute quantification. Flow stability and repeatability are essential here, which is why integrated designs often rely on consistent routing and switching via solenoid valves and controlled delivery with compact pumps.
If PCR is part of your roadmap, it is typically most efficient to start from a chip architecture built for scalable integration: microfluidic chips.
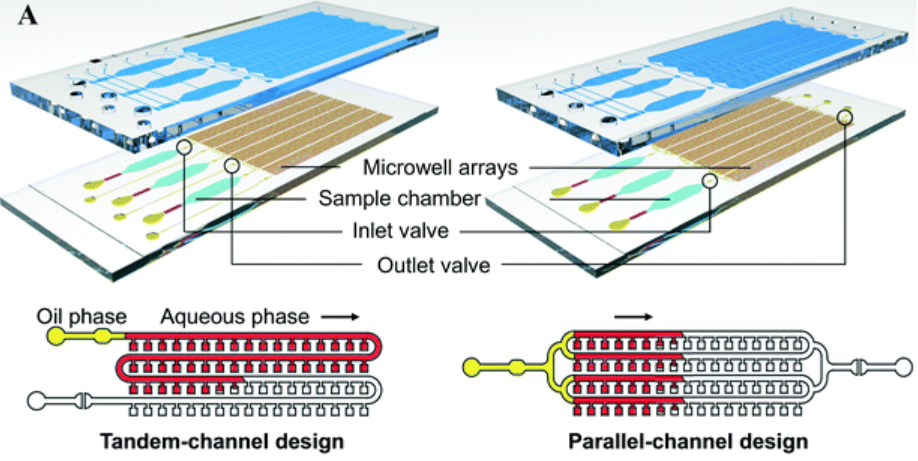
Microfluidic Chips for Polymerase Chain Reaction (PCR)
Pathogen detection demands speed and reliability—often under time pressure and with varying sample quality. Microfluidic systems can integrate sample handling and detection steps into a streamlined workflow, reducing turnaround time and simplifying operation.
This is especially valuable in decentralized testing environments where consistent performance matters but specialist operators may not be available. In these platforms, stability often comes from reducing connection points and enabling repeatable routing using integrated structures like bonded manifolds.
Wearable microfluidics is advancing quickly because it provides a practical pathway to non-invasive monitoring. Sweat is a widely used biofluid, and flexible microfluidic patches can route sweat through channels to enable real-time analysis or time-stamped sampling.
From an engineering standpoint, wearable microfluidics places extra demands on sealing and mechanical reliability—making integrated flow paths and robust packaging design just as important as the sensing method. When wearables evolve from prototypes to scalable products, integration approaches that reduce leakage and assembly variation become critical—often supported by manifold-style packaging concepts such as bonded manifolds.
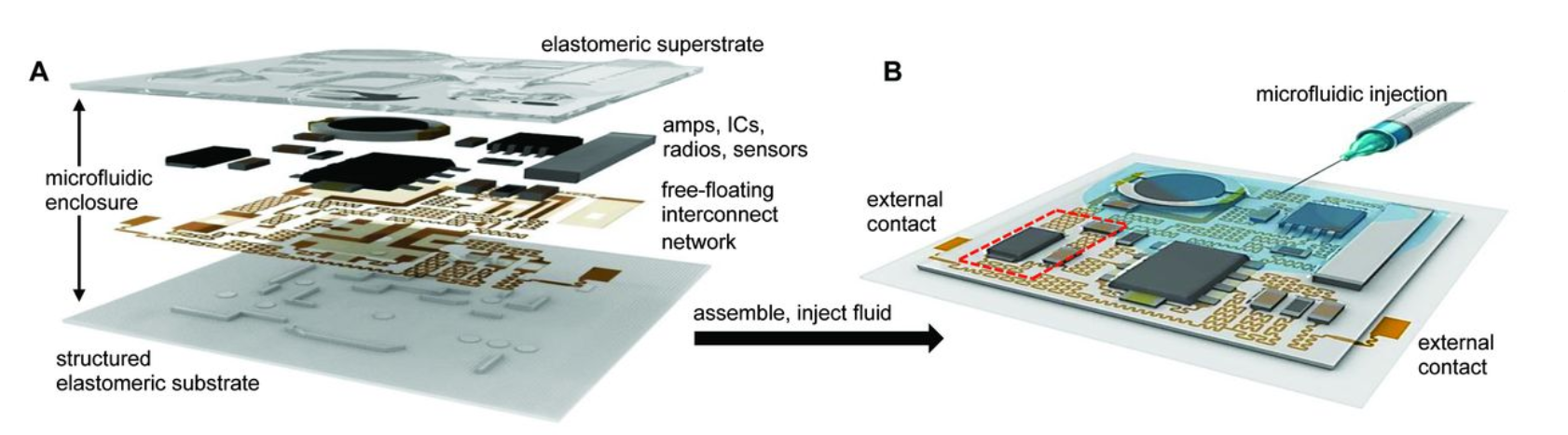
Flexible Microfluidic Devices for Skin-Based Detection
Exosomes are widely studied as biomarkers because they can reflect disease status and are present in body fluids. The key challenge is separation and preparation: biological samples are complex, and traditional workflows may be time-consuming or equipment-heavy.
Microfluidic chips offer an attractive alternative by enabling structured micro-scale operations and potentially integrating multiple preparation steps into one cartridge. Reliable control of fluid pathways is essential—particularly for multi-step workflows—so practical designs often incorporate consistent switching via solenoid valves and stable transport using compact pumps.
Organ-on-a-chip platforms use microfluidics to create controlled microenvironments that better mimic physiological conditions than static culture. These systems can support long-term perfusion, controlled exposure, and repeatable gradients—useful for mechanistic research and preclinical evaluation.
As organ-on-a-chip moves toward broader adoption, the ability to maintain stable flow paths and reduce leakage becomes increasingly important. Integration approaches using bonded manifolds can help improve robustness and assembly consistency for multi-channel perfusion designs.
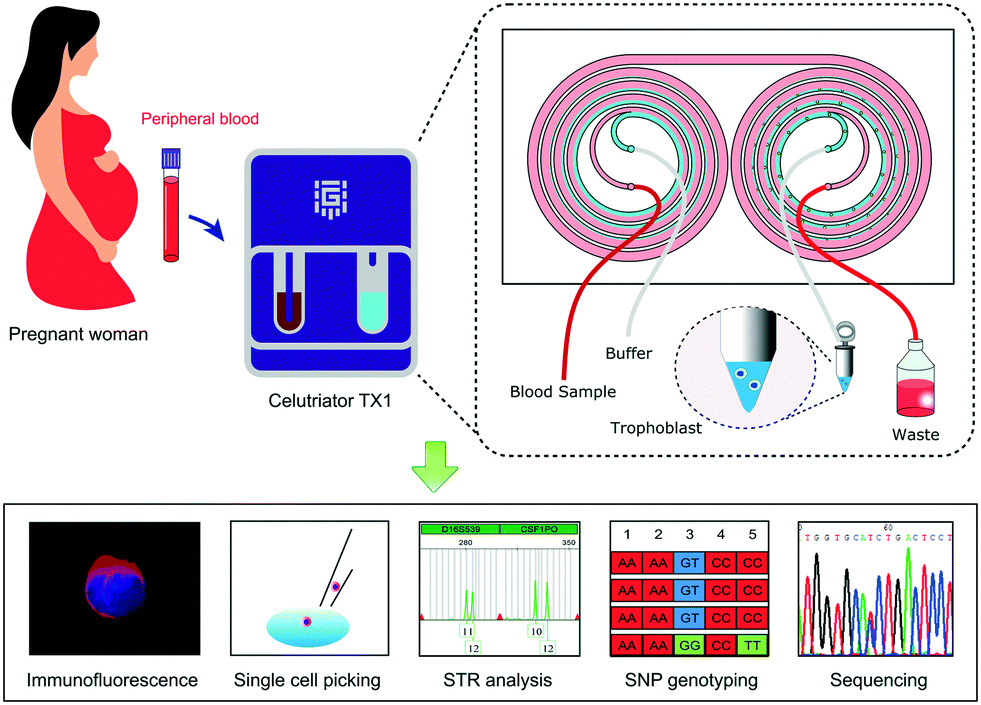
Ultra-Rapid Trophoblast Enrichment for Non-Invasive Prenatal Testing (NIPT)
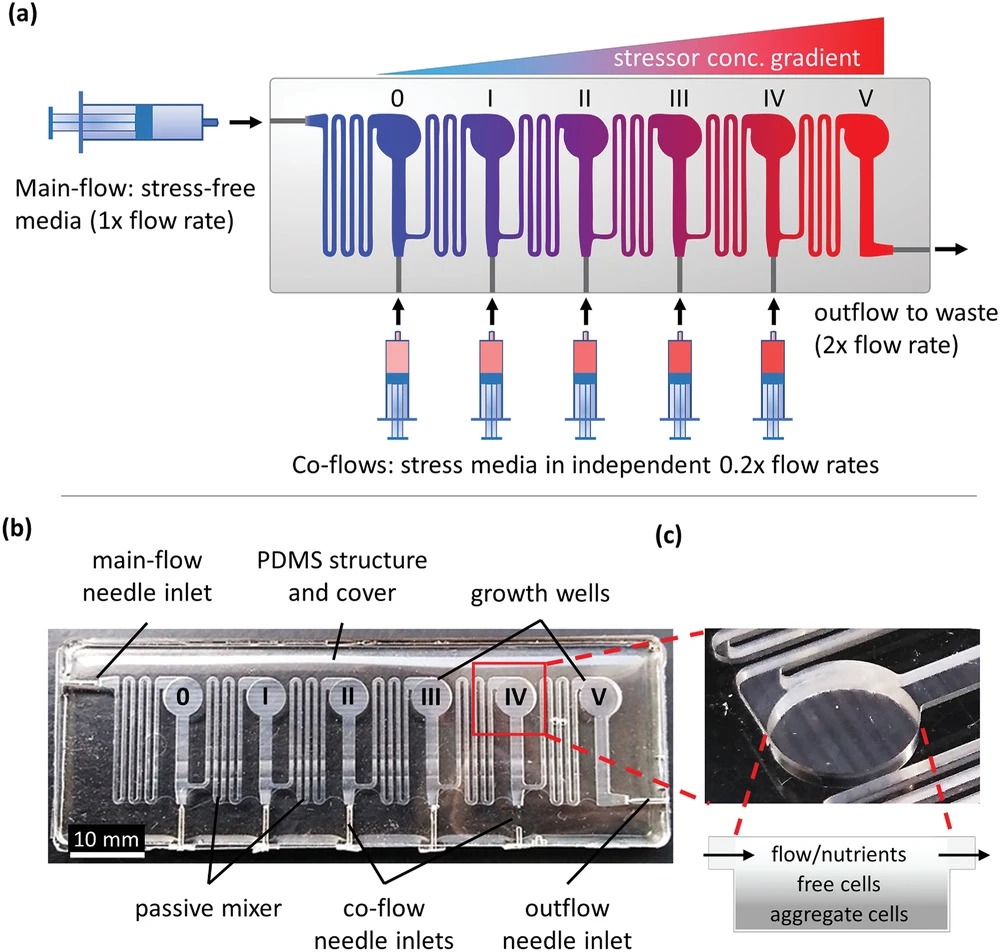
Microfluidic chips enable precise gradients and controlled exposure conditions that are difficult to reproduce reliably in macroscale setups. This supports drug screening, microbial adaptation studies, and analytical workflows that need consistent microenvironments.
In practice, these platforms depend on stable dosing and reproducible transport, which is why compact fluid delivery components—such as micro-scale pumps—are often central to performance.
Microfluidic chips are moving rapidly from “experimental prototypes” to manufacturable platforms. The strongest growth drivers include:
In real product development, success often depends less on the assay concept and more on system-level engineering: sealing, flow stability, repeatable switching, and manufacturability. A practical pathway is to align the platform architecture early—linking microfluidic chips with integration structures like bonded manifolds and reliable control components such as solenoid valves and compact pumps.
If you are developing a portable diagnostic cartridge, a lab automation module, or an integrated analytical device, these pages may be relevant: