-
Home
-
Products and Solutions
-
Keyto Global
-
News Center
-
Service Support
-
About Us
-
Contact Us
-
语言


Designing a compact, reliable, and compliance-ready fluid control system is increasingly challenging for medical devices, IVD analyzers, POCT platforms, biopharma instruments, and laboratory automation systems. Traditional decentralized fluidic architectures—assembled from separate micro valves, pumps, tubing, and fittings—often add engineering complexity, increase leak-risk interfaces, and slow down verification and documentation.
Keyto Integrated Manifold Modules consolidate critical fluidic functions into a single, precision-engineered integrated fluidic manifold. By moving complexity upstream into an engineered module, instrument teams can reduce integration burden, speed development, and improve system-level consistency across builds.

If you are building an instrument where repeatability, stability, and traceability are non-negotiable, the fluidic subsystem often becomes the bottleneck—especially when the design relies on many discrete parts and interconnects. An integrated manifold module addresses this by combining routing and functional components into a compact architecture that is easier to validate, manufacture, and service.
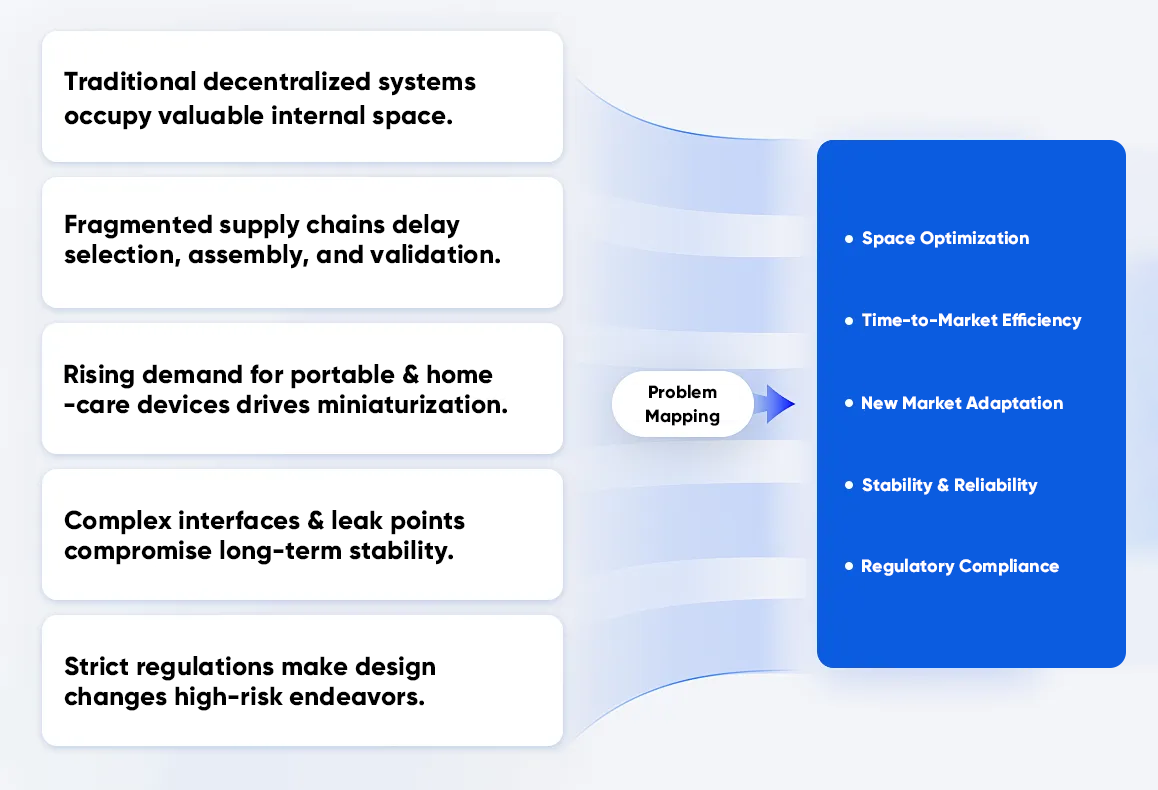
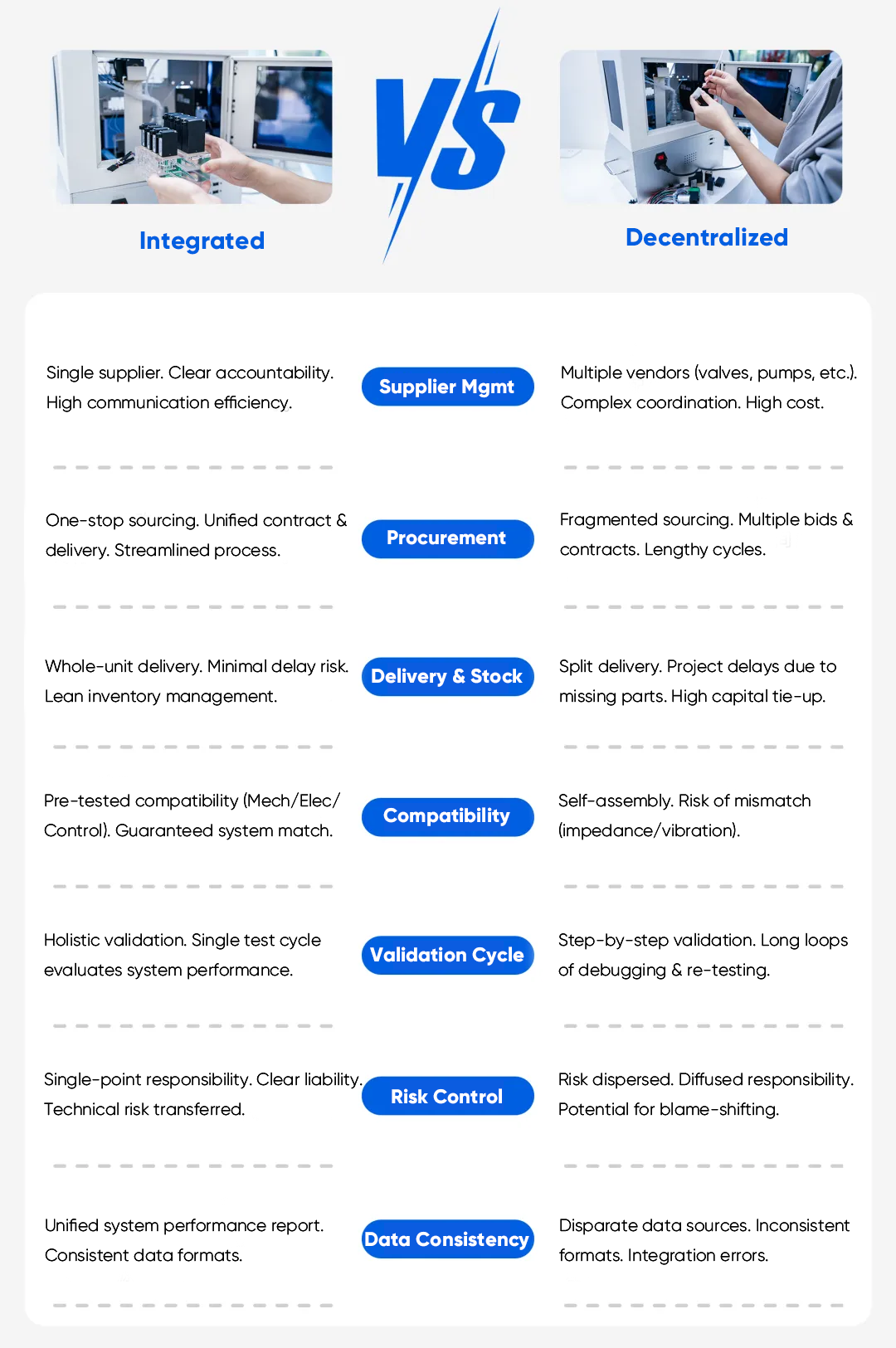
Decentralized designs can work, but they frequently create system-level issues that become costly as you scale from prototype to production. These problems show up most often in POCT, IVD, and lab automation projects where space is limited, reliability targets are strict, and changes must be carefully controlled.
Portable and benchtop instruments have limited internal volume, and discrete fluidic components compete directly with optics, thermal control, electronics, and mechanical structures. Multiple tubing runs and adapters also restrict layout flexibility and can complicate thermal or vibration considerations. In practice, the fluidic layout becomes a packaging problem as much as a functional one.
Multi-vendor sourcing introduces tolerance mismatch, mixed documentation standards, and longer selection cycles. Even when each component meets its own specification, the assembled system can drift due to stack-up effects across interfaces. That variability increases the cost of assembly, testing, and troubleshooting, and it can slow the path to a stable production configuration.
Market demand continues to push toward smaller form factors. As instruments shrink, there is less tolerance for long tubing paths, large dead volumes, or scattered components. A compact microfluidic manifold approach becomes a practical requirement when you need predictable routing and controlled internal volumes.
Every fitting, gasket, tube joint, and adapter is a potential failure mode. More interfaces typically mean more leak paths, more opportunities for trapped air or contamination, and more points to inspect during service. In regulated or clinical environments, the downstream cost of instability can be significant: failed runs, increased maintenance, and inconsistent performance across sites.
Design changes in regulated programs often trigger documentation updates across verification, validation, traceability, and manufacturing controls. A fragmented fluidic BOM can expand the scope of documentation management. A more integrated architecture can simplify configuration control because a larger portion of the fluidic subsystem is defined and validated as a single module.
An Integrated Manifold Module is a compact, engineered fluid control module that combines multiple components—such as micro valves, micro pumps, sensors (as needed), fittings, and defined internal routing—into a single manifold-based subsystem. Instead of assembling and validating a large set of discrete parts, teams integrate a pre-configured fluidic core designed around application requirements.
Conceptually, it is a shift from “component management” to system-level fluidic integration. The goal is not simply to mount parts on a block; it is to define a stable fluidic architecture that is easier to assemble, validate, replicate, and maintain across the product lifecycle.
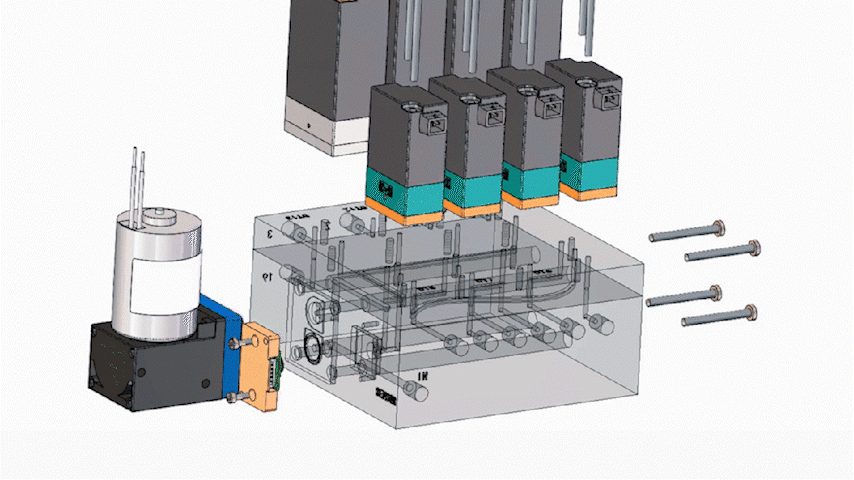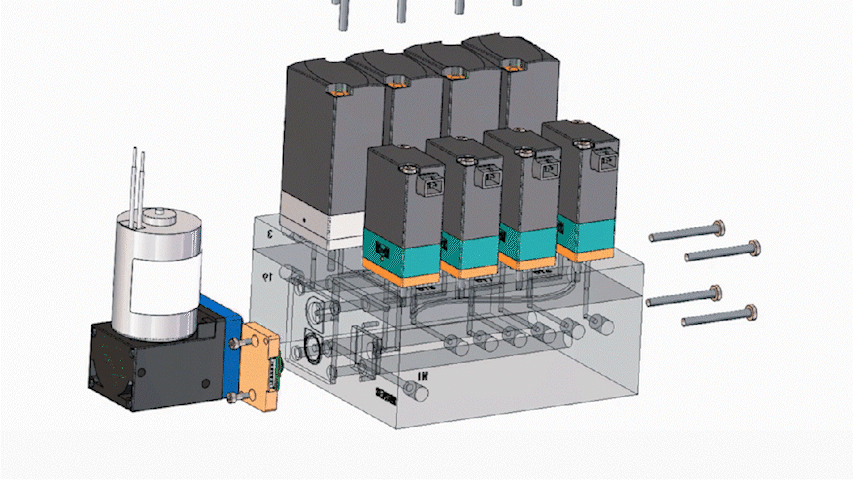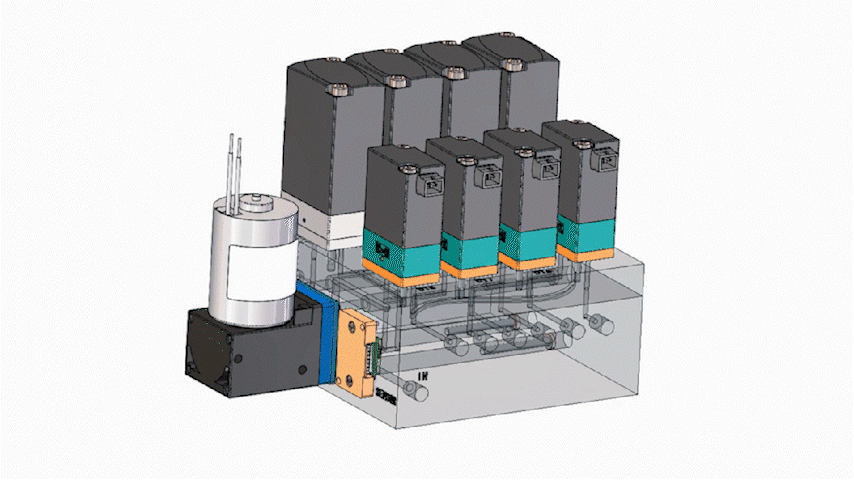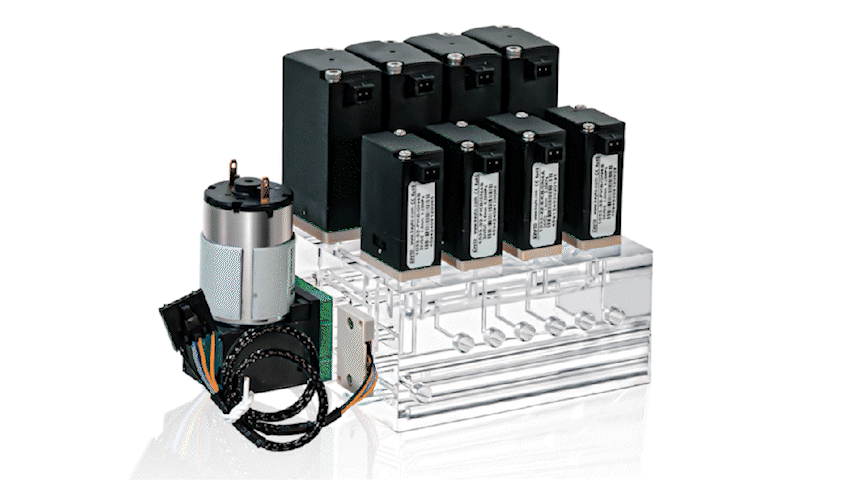
In miniaturized instruments, space is a premium. Consolidating multiple fluidic interfaces into a single integrated structure can significantly reduce routing complexity and reclaim internal volume for core subsystems such as optics and thermal control. Many programs see meaningful footprint reduction after integration, although outcomes depend on routing, channel count, and service constraints.
Beyond size reduction, integrated routing can also improve design clarity. Instead of managing multiple tube paths and fittings across the chassis, the fluidic functionality is centralized into a defined module with consistent port locations and internal channel geometry.

Development speed typically slows when teams iterate through repeated sourcing, assembly, leak testing, and calibration of decentralized builds. An integrated module can shorten these cycles by allowing configuration around application parameters such as flow range, pressure, media compatibility, port geometry, dead volume targets, and cleaning or sterilization requirements.
When the module is engineered as a coherent unit, teams spend less time reworking tubing, fittings, and interface decisions. That enables R&D to focus on system differentiation—assay performance, control logic, user experience, and instrument architecture—rather than repeated fluidic rebuilds.
For medical and biopharma-adjacent instruments, stability is a baseline expectation. Integrated manifold modules improve reliability primarily by reducing external interfaces and creating a more controlled routing architecture. With fewer joints and adapters, there are fewer places for leakage, drift, or installation variability to appear.
Integration can also improve lot-to-lot repeatability because more of the fluidic network is defined by a single engineered structure, rather than by technician-dependent tubing paths and connector handling during assembly.
Keyto supports material options aligned to common requirements in medical devices and lab instruments, such as medical-grade polymers, stainless steel, and other specialty materials depending on chemical compatibility and process constraints. Material choice is typically driven by media exposure, cleaning method, sterilization requirements, and any biocompatibility or extractables considerations for wetted paths.
For many instrument teams, documentation readiness is as important as mechanical performance. Keyto can support customer QMS workflows with deliverables such as engineering drawings and specifications, applicable test/validation reports, and material certifications or traceability documentation—structured to reduce friction during audits and internal reviews.
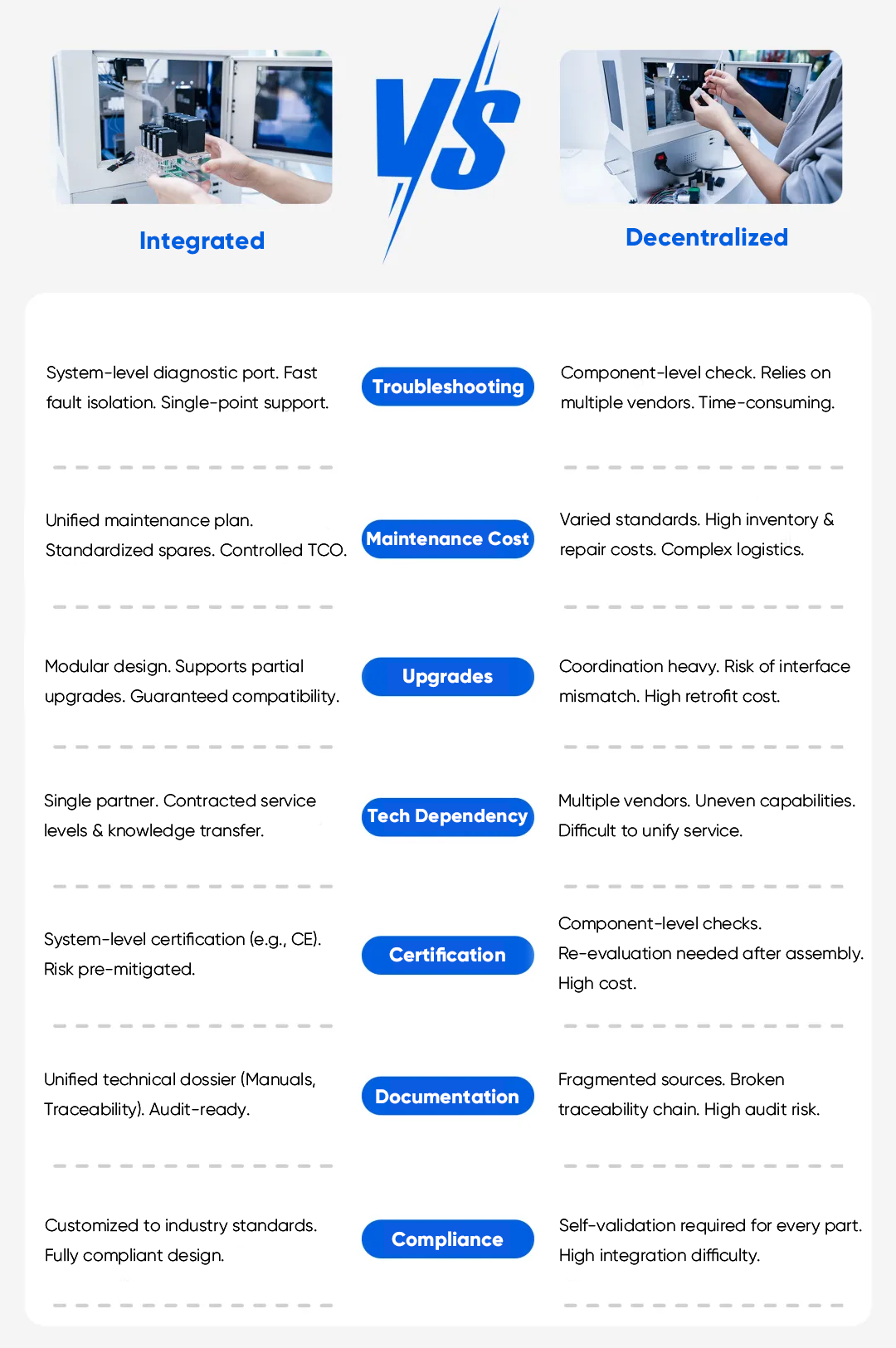
Lifecycle efficiency directly impacts uptime and total cost of ownership. In decentralized systems, field troubleshooting can be slow because faults may originate from any number of fittings, tube joints, or discrete components. Integrated modules can simplify service strategy by centralizing the fluidic core and making replacement more predictable.
In many instrument programs, a module-based approach supports faster restoration of performance: replace the module, confirm function, and return the instrument to operation. When paired with standardized or customized quick-connect interfaces, maintenance workflows can be easier to train, easier to document, and less prone to variability across service teams.
Integrated manifold modules are particularly valuable in IVD and clinical workflows where contamination control, repeatability, and stable fluid routing matter. Typical use cases include sample preparation, reagent dispensing, washing steps, and multi-step fluid sequencing—especially when the instrument must maintain consistent performance across high volumes and multiple sites.
Integration can support low-dead-volume routing concepts where appropriate, reduce carryover risk by minimizing interfaces, and simplify configuration control when assay workflows evolve over time.
In biopharma-related development and processing environments, the fluidic architecture often needs precise dosing, controlled flow, and strong contamination defense. Integrated modules can help address sterile connectivity challenges and support consistent batch-to-batch performance, particularly when repeatability and documentation are emphasized.
In lab automation—especially high-throughput sample handling and injection workflows—fluidic stability directly affects throughput and data integrity. Integrated designs can support parallel channel architectures, reduce response variability, and provide more predictable routing for repeated cycles in automated workflows.
A typical engagement begins with technical alignment around the instrument workflow and the fluidic requirements that matter most for performance and compliance. This usually includes the target application and use-case sequence, media and contamination constraints, flow and pressure requirements, footprint goals, port locations, dead volume considerations, reliability targets, and documentation expectations aligned with your QMS.
From there, Keyto proposes a module architecture and integration strategy designed to reduce engineering burden and accelerate time-to-market—while keeping manufacturability, traceability, and service strategy in view from the beginning.
Contact Keyto to discuss your custom integrated manifold module requirements and receive a preliminary technical assessment.
A valve manifold typically focuses on mounting and routing for valves. An integrated manifold module is a broader fluid control module that can combine valves, pumps, sensors (as needed), fittings, and validated routing into a plug-and-play fluidic core designed around a specific workflow.
No. Medical/IVD and biopharma applications benefit strongly due to reliability and documentation requirements, but integrated modules are also widely used in laboratory automation, analytical instruments, and other precision fluid handling equipment.
Common inputs include target flow rate, pressure range, fluid media, port configuration, footprint constraints, dead volume targets, reliability requirements, and documentation or traceability expectations.
In many designs, yes. Integration generally reduces the number of external tubing joints and connection interfaces, which are common leakage points in decentralized assemblies.