+86-755-29516669
- Gallery |
- VR View |
- OEM Service |

Recently, there have been frequent news of winning bids in the field of in vitro diagnostics.
Speaking of in vitro diagnostic lines, originating in Japan and later developed in Europe and the United States, it introduces the concept of industrial automation into laboratory testing, connects testing systems and processing modules with rails, and reduces the overall testing time. In the background of medical innovation, we can see more and more IVD enterprises entering the pipeline market.
As a specialized enterprise in the field of fluid control, Keyto has provided high-quality supply services to its customers in multiple systems such as pre-processing units, analysis and testing units, and post-processing units in the in vitro diagnostics industry, and has reached deep cooperation with many leading enterprises in the in vitro diagnostics industry.
Common assembly line systems are divided into two levels: "Modular Automation (MA) and Total Laboratory Automation (TLA) "According to the classification of testing substances, assembly lines can be divided into biochemical immune workstations, hemostasis and blood analysis workstations, molecular diagnostic workstations, urine analysis workstations, and microbiology workstations, etc.
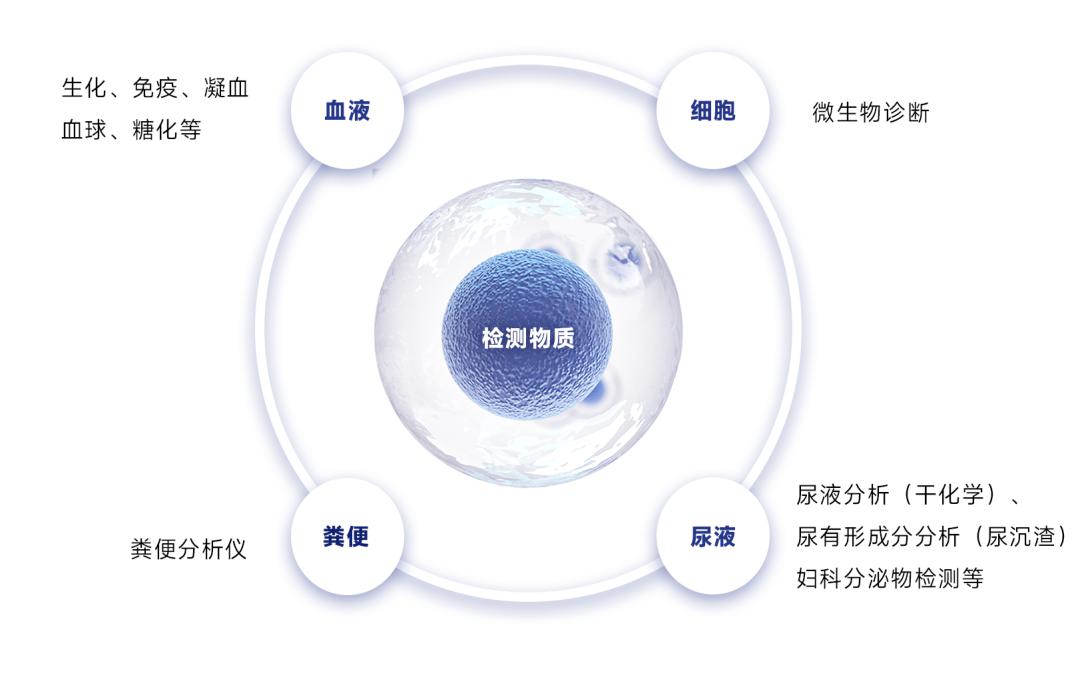
An automated assembly line can achieve complete automation of the entire process of sample sorting, transportation, processing, analysis, and storage. The assembly line not only has a fast testing speed and high efficiency but also greatly reduces errors and cross-contamination caused by manual operations. The testing results are more stable, reliable, efficient, and of high quality.

To facilitate a more intuitive understanding of the basic operational principles of the assembly line, it can be differentiated into three attribute directions: fluid circuit, gas circuit, and electrical circuit. These attributes can further be subdivided into functional areas such as mechanical structure, analytical detection, temperature control, control, and reagent areas. By mapping these functions to the principles of the instruments, the complexity is mitigated.
The in vitro diagnostics industry market features a wide variety of instruments with varying functionalities and timelines for each manufacturer. This is a typical scenario characterized by diversity, customization, and long product lifecycles. Keyto, considering commonalities and specific attributes of each customer, develops and supplies compatible solutions to ensure precise service alignment with the end-user's requirements.

Throughout the collaboration with customers, Keyto maintains a constant focus on providing excellent, professional, and standardized services. Compatibility is ensured during the collaboration period, covering aspects such as customer terminal environment, instrument types, selection requirements, installation methods, reagent types, and operational applications, thereby reducing compatibility errors, as well as additional trial and communication costs.
In the in vitro diagnostics assembly line model, the relationships between each instrument are inseparable. There is a significant need for coordination among fluid circuits, gas circuits, and electrical circuits. Handling these interconnections requires a considerable amount of time, effort, and personnel for component selection and visits to supply companies.

Keyto, through long-term cooperation with customers, has accumulated extensive selection experience and technical capabilities. They have introduced eight major categories with nearly 5000 different specifications of products, strategically locating manufacturing bases in multiple places in Shenzhen to enhance overall supply capability.
Case 1: Complex Requirement, High Trial Costs
Using a simplified simulation of a common automated blood analysis pipeline in in vitro diagnostics as an example, Keyto addresses the complexity of selection. The diagram below is for learning reference only; actual applications may vary. For related needs, consultation with our sales personnel is recommended for personalized selection and adaptation based on your instrument.

Case 2: How to Meeting Reliability Requirements
In actual operational conditions, as the assembly line instruments process numerous samples per hour, supporting continuous uninterrupted sampling, the demands on the lifespan and conditions of components become stringent. This necessitates that supply companies possess quality control capabilities in real-world applications. Keyto provides relevant solutions in research and development, quality control, and other aspects to ensure product reliability under actual working conditions.
Solution Capabilities:
Provide a specialized development mode based on the user's actual application needs.
Rigorous reliability verification experiments, simulating aging, environment, temperature, mechanical stress, salt spray, reagents, etc., to ensure reliability under actual working conditions.